Which of the Following Elements Has the Lowest Electronegativity
119 rows ELEMENT ELECTRONEGATIVITY. Element A has an electronegativity of 08 and element B has an electronegativity of 30.
Which Of The Following Elements Has The Lowest Electronegativity Class 12 Chemistry Jee Main
Centrally located directly between.

. Of the following elements which has the lowest electronegativity A Br B Mg C Ca from CHM 1045 at Florida International University. Based on the indicated electronegativities arrange the following in order of increasing ionic character. 89 - Atomic number.
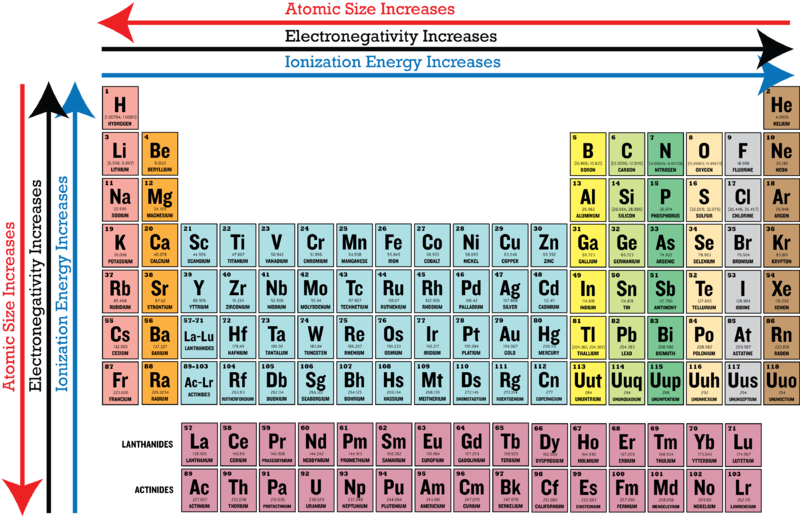
The most electronegative of all elements are fluorine. Group 18 elements have no electronegativity. 90 - Melting point.
The chemical elements of the periodic chart sorted by. If we see the four options li have one valence electron in its valence shell and it always tends to lose that electron to attain the stable configuration and shows least electronegativity whereas in the next option is carbon here carbon have a valency of 4 br has 7 valence electrons and f also has 7 valence electrons and these three atoms can. Of all the Group 13 elements the element with lowest electronegatively is.
Carbon has a larger radius and thus exerts greater control over the shared electron pair bcloser to F because fluorine has a higher electronegativity than carbon ccloser to C because carbon has a lower electronegativity than fluorine d. So iodine has the lowest electronegativity. Its electronegativity is 40.
Which of the following elements has the lowest electronegativity. An inadequate model because the bond is ionic e. Therefore Fluorine is the most electronegative element and cesium is the least electronegative element.
In the valence shells of lithium carbon bromine and fluorine lithium is the only element where it is more disadvantageous to gain electrons instead of losing electrons as to gain a full outer shell. Electronegativity increases as you go further to the right of the periodic table and closer to the top of the periodic table. Asked Oct 17 2020 in Chemistry by izzyme.
The Periodic Table contains a lot more information than merely the names of each of the chemical elements. High ionization energy low electronegativity high reactivity. The lowest electronegativity of the element from the.
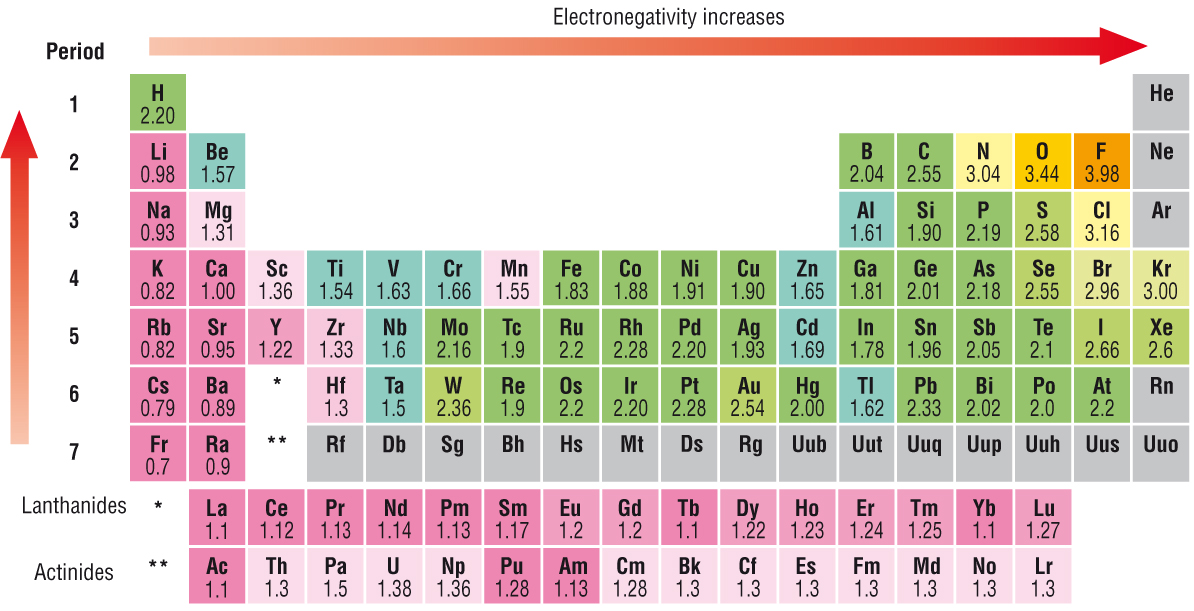
Metals have electronegativities less than 20. Element Electronegativity Br 28 P 21 Mg 12 La 10 Cs 07. Which Of The Following Elements Has The Lowest Electronegativity Class 12 Chemistry Jee Main What Does The Electronegativity Of An Element Indicate Quora Solved Of The Following Elements Which Has The Lowest Chegg Com What Is Electronegativity Chart List Of Electronegativity Pdf Elements Following Has wallpaper.
The angle between the bonds in CHs is approximately a. In the case of carbon bromine and fluorine they all can gain electrons. CsBr LaBr3 PBr3 MgBr2.
The electron pair in a bond could be considered acloser to C. Chemistry questions and answers. Of all the Group 13 elements the element with lowest electronegatively is.
Find an answer to your question Which among the following has the lowest electronegativity. In moving down to the halogen group EN. The noble gases ie.
ALi b C d o 10. Hence option D is correct. The least electronegative elements are cesium Cs and francium Fr with electronegativity values of 07.
Decreases hence Iodine has lowest electronegativity. A K B Rb C Br D Te E I 4. The element with the lowest electronegativity in Period 3 is - answer choices.
A key piece of information they contain is the. Which of the following generally applies to the noble gases. 19 - Atomic Mass.
Fluorine has the highest electronegativity. Atomic number - Name alphabetically. Which of the following elements has the lowest electronegativity.
The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values of elements from 0 to 4. High ionization energy high electronegativity high reactivity. As such it has a lower tendency to attract electrons and thus has a lower electronegativity.
Fluorine has the highest electronegativity followed by chlorine bromine and then with the least reactivity we have iodineamongst the given options. The molecule CHa has a tetrahedral shape. Which of the following elements has the lowest electronegativity.
Element A has an electronegativity of 08 and element B has an electronegativity of 30. So iodine has the lowest electronegativity. Which statement best describes the bonding in A3B.
This is because they already have eight electrons in their outermost shell and.

Which Of The Following Elements Has The Lowest Electronegativity How Do You Know

Lesson 13a Periodic Trends Chemistry Quiz Quizizz
What Would Cause An Atom To Have A Low Electronegativity Value Socratic
Comments
Post a Comment